
European citizens against animal experimentation

The European Citizens' Initiative (ECI) proposes strengthening the ban on the use of animals in cosmetics and extending it to cover chemicals and research in general.
The European Citizens' Initiative (ECI) ‘Save Cruelty Free Cosmetics - Commit to a Europe without Animal Testing’, supported by more than one million European citizens, was submitted to the Commission at the end of January 2023. As part of its response, the Commission organized a workshop on 11th and 12th of December 2023, bringing together legislators, representatives of associations and scientists to discuss the various steps needed to develop a roadmap for replacing animal testing in chemical safety assessment (effects on human health, on the environment, etc.).
Contextualization of the roadmap
Following the project introduction, the various stakeholders were given the opportunity to express their perspectives and expectations regarding the roadmap. Through short presentations, they shared the state of the art in their fields, mentioned ongoing projects and highlighted key points that needed further consideration.
Jay Ingram, from Humane Society International, one of the non-governmental organizations (NGOs) behind the ECI, emphasized the need to question existing regulatory frameworks and to adopt a holistic perspective on protecting human health and the environment. He also raised the issue of the semantics of the "NAMs" acronym - which can refer to "New Approach Methodologies" in its original sense, or to "Non-Animal Methods" - highlighting the importance of terminology for effective communication.
Carl Westmoreland, Director of Science and Technology in Unilever's Environmental Safety Division, presented the EPAA (European Partnership for Alternative Approaches to Animal Testing) 'NAMs Designathon' initiative, which aims to use data from NAMs to classify chemicals according to their risk of systemic toxicity to humans.
This classification would be based on two criteria :
-
Intrinsic toxicodynamic: the potential toxic activity of the compound (cell morphology, transcriptomics, etc.)
-
Toxicokinetic properties: the bioavailability of the compound after exposure (Absorption, Distribution, Metabolism, Excretion, physiology-based kinetic, etc.) to reflect the "level of concern" (High, Medium or Low) associated with each chemical.
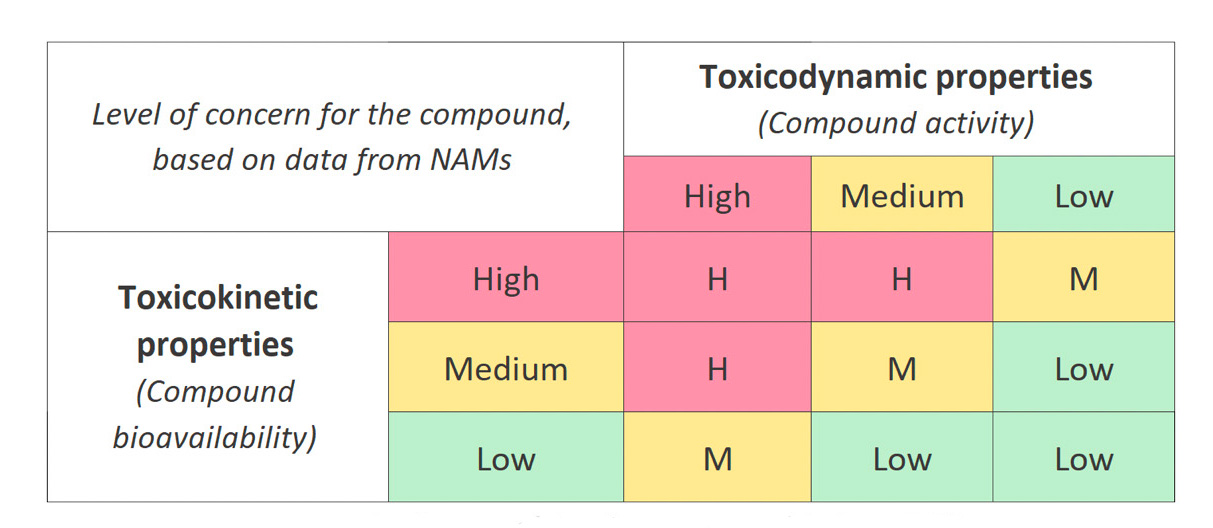
A pilot study of 150 chemical compounds is currently underway to refine this classification, and a workshop will soon be held between its participants to compare the different methods and identify which NAMs are most effective for classifying the different types of chemicals.
Adam Lilicrap, Director of Research in Ecotoxicology and Risk Assessment at the Norwegian Institute for Water Research (NIVA), focused on the intensive use of fish in aquatic toxicity tests for environmental protection.
The OECD Test Guideline TG 203 uses death as the criterion for acute toxicity. Replacement methods already exist, such as the OECD Test Guideline TG 249 on the "RTgill" rainbow trout gill cell line, validated in 2021, or QSAR (Quantitative Structure-Activity Relationship) tools. Another method – OECD Test Guideline TG 236 - is based on the use of fish embryos rather than adults. Adam Lilicrap also underlined the limitations of ecotoxicology tests, which are carried out on only a few species and are not representative of the environment as a whole.
Focus on regulatory issues
The focus on regulatory aspects led to a reflection on working methods. Emphasis was placed on the processes for validating NAMs, on building confidence and on the regulatory acceptance of NAMs. It raised the question of whether the various regulatory tools (legislative, OECD, etc.) can still accurately reflect the current state of the science.
Christophe Rousselle, risk assessor at ANSES, shared his experience and perspective on the use of NAMs in risk assessment. He stressed the importance of providing transparent and detailed data on the reproducibility, sensitivity and specificity of NAMs when a dossier is submitted. Currently lacking, these informations would help educate assessors and build strong confidence in NAMs. As deputy coordinator of the PARC project (Partnership for Risk Assessment of Chemicals), he also presented ANSES's involvement in this project, and in particular in Work Package 5, which is specifically dedicated to hazard assessment using NAMs.
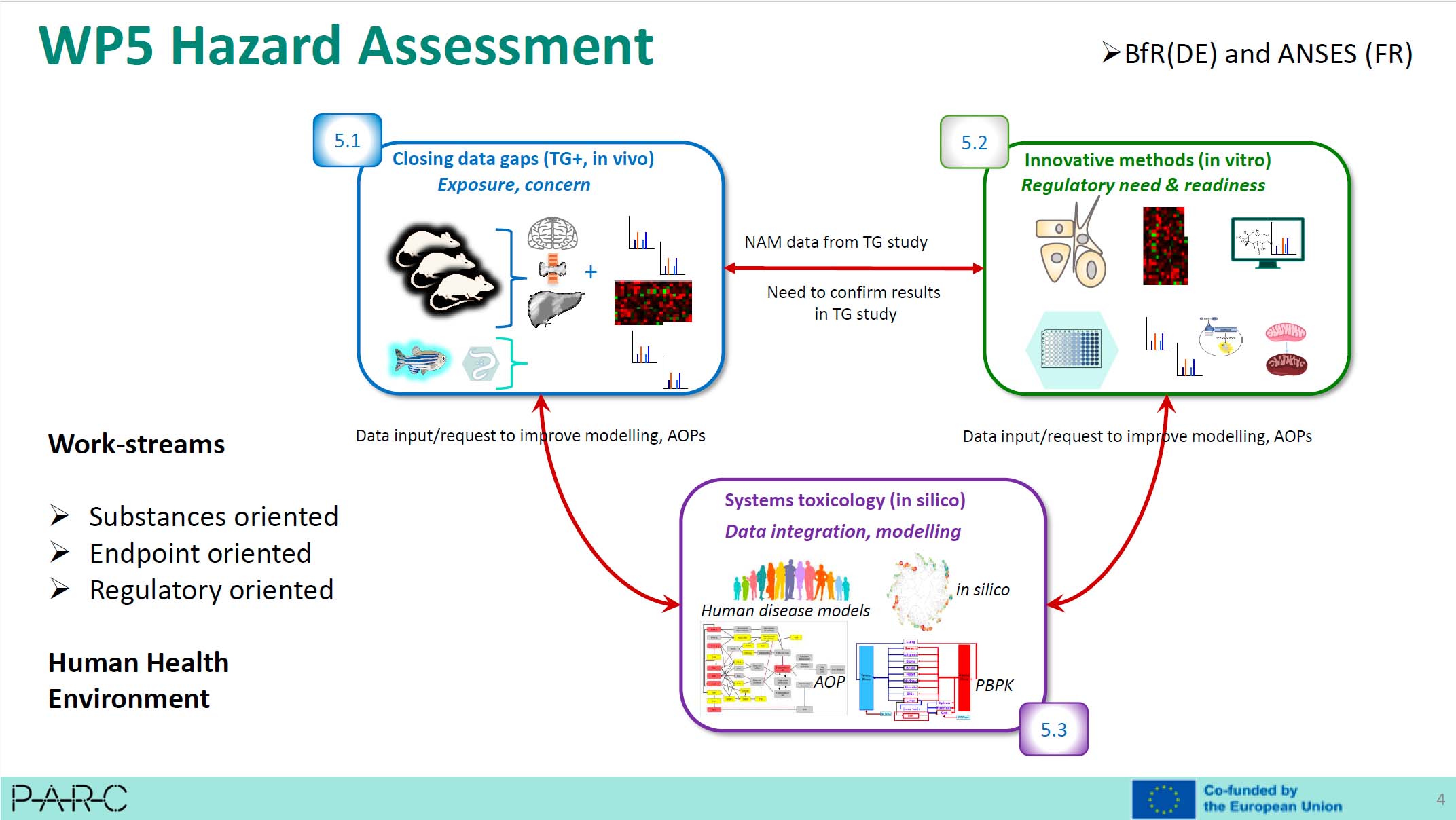
João Barroso, Scientific Officer at EURL ECVAM, discussed the various aspects and points of evolution of the OECD's Guidance Document GD34, which guides the validation and international acceptance of new risk assessment methods. Drafted 20 years ago, the actual version of this document no longer reflects the current state of scientific knowledge. The main focus of the revision will include the validation of "Defined Approaches", the integration of practical guidance on validation, the definition of the concept of technical validation, the assessment of the relevance of a NAM and the validation of artificial intelligence and organ-on-a-chip. The revised version of the document is expected to be discussed within the OECD in April 2025.
Harmonization of language, precise definition of objectives, international collaboration and communication, examination of the regulatory context, and addressing the validation aspects of NAMs will be the key elements of the roadmap. It is evident that a paradigm shift, both societal and regulatory, is required to implement these methods stemming from a constantly evolving science.
A forthcoming workshop scheduled for late 2024 will assess the progress made in developing this roadmap.
