The digital beagle

The "virtual dog" project, designed to replace the use of dogs in toxicity studies, was recently co-funded by NC3Rs and the pharmaceutical industry to the tune of £1.6 million.
A digital twin is a virtual equivalent of a physical object or system, "fed" with already existing data, which allows to conduct important simulations and predictions, and obtain results that were unreachable by other means.
In life sciences, the evolution of digital twins able to simulate biological systems with high fidelity presents an innovative paradigm, promising endless possibilities and major changes in research methodologies. Digital twins also harbor the potential to bring forward a more ethical research by facilitating the Replacement and Reduction of animal experiment.
Digital pathways to ethical research: empowering the 3Rs
By offering a platform for predictive modeling and conducting in silico experiments, these twins allow for virtual testing of hypotheses, drug responses, and disease mechanisms, thereby minimizing the number of animals required for experimentation in certain scenarios.
This preselection process, ensuring that only the most promising avenues are pursued in animal research, will help Reducing overall animal usage while enhancing the quality and relevance of the studies conducted.
Moreover, by being able to accurately model biological systems at various scales, replicating physiological and pathological processes within a controlled digital environment, these numerical twins could play a pivotal role in Replacing animal experimentation, as they could allow researchers to acquire a detailed understanding of complex systems without resorting to live animal experimentation.
Three digital twin projects carried out by Inria have been presented at the “Digital tools” EMBO conference that took place in Sant Feliu de Guixol (Spain) in November 2023.
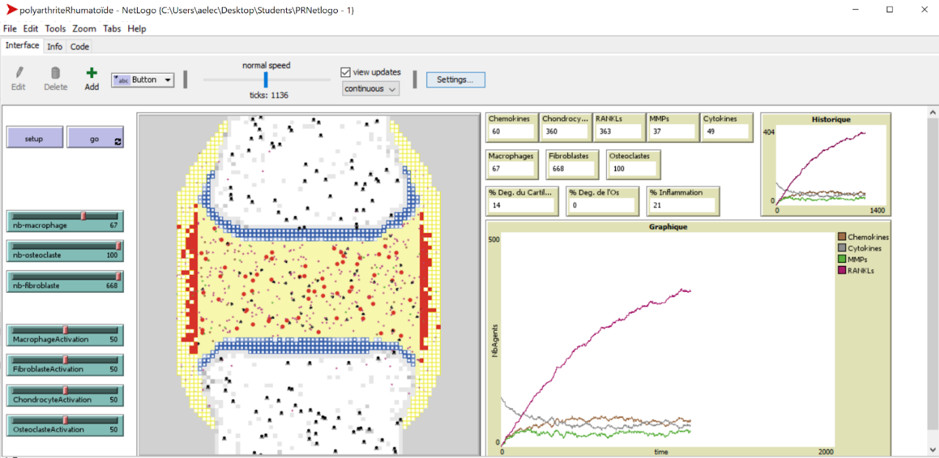
A Digital twin for Rheumatoid Arthritis: Science or science fiction?
Dr. Anna Niarakis (University of Toulouse III-Paul Sabatier, Inria Saclay) is pioneering the development of a revolutionary digital twin model specifically tailored for Rheumatoid Arthritis (RA). This autoimmune disorder triggers multifaceted joint inflammation, affecting patients where conventional antibody treatments are ineffective in approximately 40% cases.
Dr. Niarakis’s groundbreaking work focuses on constructing a dynamic 'digital joint,' aiming at simulating the disease's progression, diverse treatment responses, and the potential risks associated with novel pharmaceutical therapies (Laubenbacher et al., 2022). Leveraging bulk and single-cell omics data, logic-based and data-driven modelling techniques, she has been working on constructing a cellular-level joint representation, intricately capturing the interactions between immune and resident cells, inflammation processes, and cartilage integrity.
Through a collaboration with Sanofi R&D, this effort has culminated in one of the most comprehensive multi-cellular models up to date (>1000 biomolecules), and the identification of promising therapeutic targets and combinations (Singh et al., 2023 ; Zerrouk et al., BioRxiv).
Dr. Niarakis's current endeavors involve the enrichment of the RA model with more cell types through the development of an agent-based model, and methods to visually correlate this wealth of data with actual patient imaging, providing a critical bridge between simulated models and real-world clinical observations.
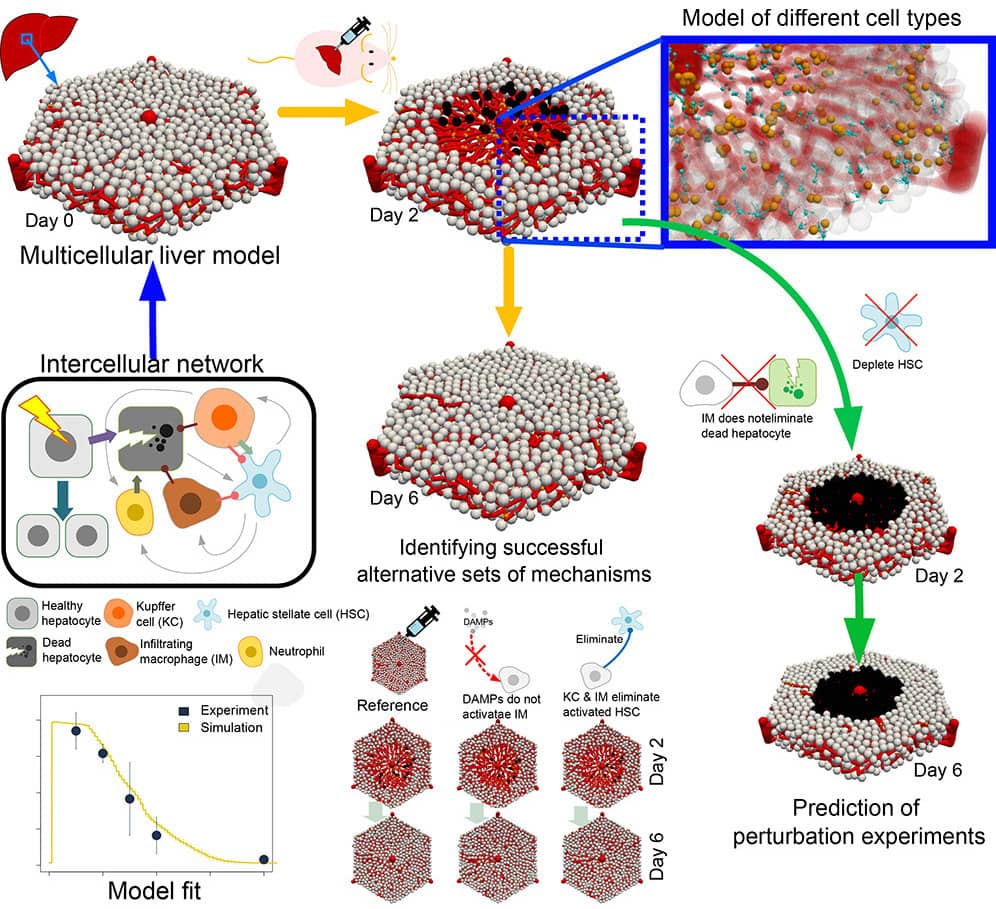
Towards a full digital liver twin: drug injury, regeneration and disease progression
Dr Dirk Drasdo, from Inria Saclay Île-de-France, and his coworkers are actively involved in developing a digital twin for the liver, a complex organ that plays a crucial role in various bodily functions, including metabolism, detoxification pathways, and glycemia regulation.
His focus lies in crafting a digital counterpart of the liver to investigate the complexities surrounding paracetamol-induced acute and chronic liver damage (Zhao et al., 2023). The mathematical mechanism-based model of the liver conceived by Dr. Drasdo and his coworkers is to serve as an invaluable tool, enabling in-depth exploration into liver damage mechanisms, examination of tissue regeneration processes, and the comprehensive assessment of consequences arising from tissue damage, by allowing the simulation of alternative hypotheses about the interplay of different cell types on regeneration.
By simulating the liver lobules’s microarchitecture using digital representations derived from imaging data, Dr. Drasdo's innovative approach takes a step further in facilitating the understanding of liver dynamics from the molecular up to the tissue scale.
Remarkably, the predictive capabilities of this model have already demonstrated compatibility with experimental data, illustrating their potential as indispensable assets for biologists and clinicians.
Building the virtual human twin: an ecosystem approach
Liesbet Geris (VPH Institute, Europe) presented the European EDITH-CSA project, a collaboration between 23 partners, funded by the European Union's Horizon Europe research and innovation program, in which French Inria is involved.
The EDITH-CSA project aims to establish the European Virtual Human Twin (VHT) Ecosystem, which will provide the necessary infrastructure, tools, and expertise to develop and deploy VHTs in order to simulate the effects of treatments, interventions, and lifestyle changes on individual patients, enabling healthcare professionals to make more informed decisions about patient care.
In EDITH, French Inria contributes to roadmapping, standards, and vision, based upon its expertise at digital twin modeling in liver and heart, in patient-specific surgery modeling, and in image-based model personalization, using models at various scales such as agent (cell)-based modeling and hemodynamics models.
The first meeting on building the VHT will take place 18-19 janvier 2024 at the Institut Polytechnique in Paris.
