Watch

On January 18th, 2024, the FC3R hosted a webinar on organoid models and their applications in research. Watch it on replay !
The Institut Jacques Monod in Paris hosted a symposium on January 29, 2024, dedicated to exploring the world of cortical and spinal neural organoids, which serve as powerful tools for studying the biology of neurons and modeling various brain diseases. This symposium - organised by the enSCORE platform with the support of DIM C-BRAINS, the Université Paris Cité, the Labex “Who Am I?”, the Institut Jacques Monod, the GDR Organoïdes, Vizgen, Stemcell technologies and Medchemexpress - offered a unique opportunity for researchers to present their work, collaborate, and delve into the latest advancements in the field.
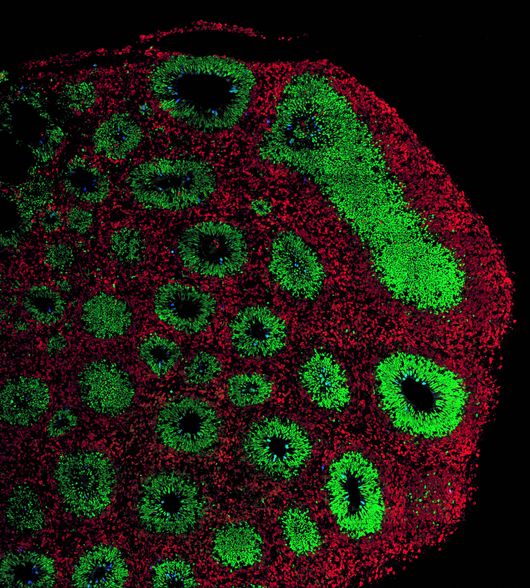
Organoids to understand and fight neurological diseases
Organoids have proven instrumental in unraveling the complexities of brain diseases, as attested by the number of talks and posters on this topic.
-
For example, Benjamin Galet (Institut du Cerveau, Paris) uses brain organoids to investigate the involvement of long non-coding RNA in the pathophysiology of Alzheimer’s disease.
-
iPSC-derived organoids allowed Miria Ricchetti’s team (Institut Pasteur, Paris) to understand that oxidative and nitrosative stress-dependant alteration of gene expression is probably a major cause of Cockayne syndrome defects, leading the way to develop new pharmacological therapies.
-
Organoids are also very helpful to study neurodevelopmental diseases, such as Joubert syndrome: Ludovica Brunetti (Institut de Biologie Paris-Seine) develops cerebellar organoids from patients to identify genes that are differentially expressed in the disease, and combines this approach with drug testing for the discovery of new treatments.
Replicating nervous system complexity
Guided protocols already enable the development of brain region-specific organoids that are highly reproducible. It is then possible to combine them in assembloids to connect different regions of the nervous system. But multi-regional organoids are also emerging :
-
Michele Bertacchi (Institut de Biologie Valrose, Nice) showcased how FGF8 treatment can influence regional identity, resulting in human cerebral organoids containing telencephalic, diencephalic, and mesencephalic-like domains.
-
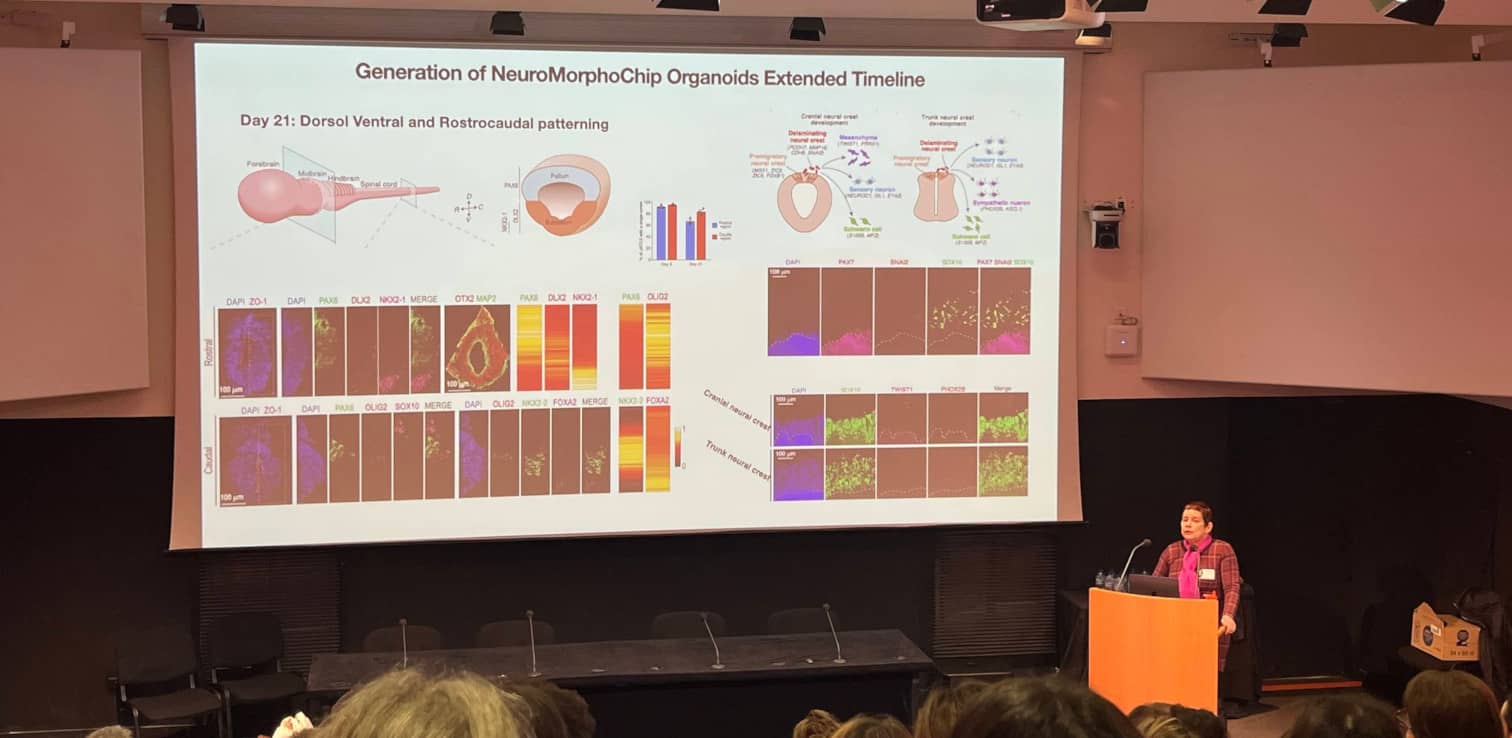
Orly Reiner (Weizmann Institute of Science, Israël) presented the NeuroMorphoChip organoids : patterned neural tube organoids derived from human pluripotent stem cells, that recapitulate the central nervous system (CNS) from head to tail. To generate these NeuroMorphoChip organoids, the cells are exposed to different morphogens gradients on chip, resulting in cell polarization, formation of a tube with a lumen with rostro-caudal patterning, followed by a dorsoventral patterning of the “forebrain”. After 21 days of development, cranial and, trunk neural crests also appear. The model can be combined with KO mutations to study the effect of specific genes on neurodevelopmental diseases, such as microcephaly.
-
Stéphane Nedelec’s lab (Institut du Fer à Moulin, Paris) has been able to induce axial elongation and neuro-mesodermal differentiation of aggregates of human pluripotent cell, to generate “spinoids” (spinal organoids) composed of a neural tube surrounded by segmented somites. Over time, the neurons differentiate in subtype according to the anteroposterior patterning, and the somites give rise to muscle progenitors that are in contact with the neural projections, creating a complex integrated trunk system to study sensory-motor circuits emergence.
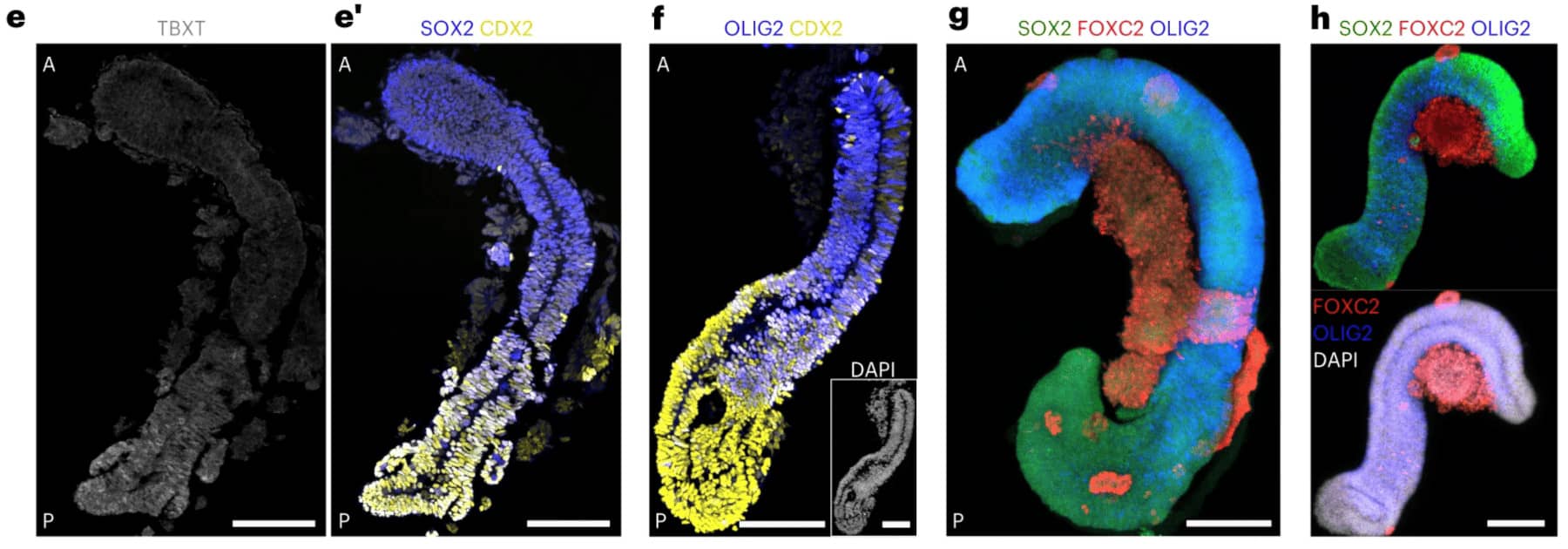
© Gribaudo et al., 2023. Spinoids” developed in Stéphane Nedelec's laboratory.
Next steps: immuncompetence and functional vascularization ?
To enhance the complexity of neural organoids, researchers are exploring the addition of other cell types, such as immune cells (microglia) and endothelial cells.
-
Hariam Raji (Institut Imagine, Paris) develops a Parkinson’s disease model of midbrain organoid from human cells, with pathological characteristics such as alpha-synuclein accumulation, and progressive dopaminergic neurons loss, and co-cultures them with iPSCderived microglia to study microglial activation proinflammatory response. The microglial cells colonize the tissue rapidly and efficiently and are able to survive within the brain organoid for at least two months. To ensure proper oxygen dissemination to the tissue, these organoids are also cultivated in a spinning bioreactor which agitates, resulting in better organoid tissue quality, maturation and differentiation.
-
Another way to ensure proper oxygenation of the tissues and allow long-term maintenance and growth of organoids would be to develop vasculature in these models. Loïc le Guennec (Institut Cochin, Paris) is developing human vascularized brain organoids with a proper brain blood barrier to study the dissemination of metastasis in the brain during T-cell acute lymphoblastic leukemia. Even if such co-cultures are already a success, challenges still reside in the ability to model a properly connected, continuous and sealed vasculature.
While challenges remain in generating complex brain models, the symposium showcased the immense potential of neural organoids, which are rapidly moving closer to replicating the complexity of in vivo organs. These models already provide unprecedented opportunities for studying biological and developmental processes previously beyond reach, such as the pathomorphology and behavior of living patient-derived neurons, or the early steps of neurogenesis. The future holds great promise for further advancements in this exciting field.
