
EQIPD webinar

To delve deeper, register for the webinar: "Enhancing quality in preclinical data - EQIPD" organized by FC3R and presented by Björn Gerlach.
The EQIPD consortium, now GoEQIPD has emerged as a key player in partnering for rigorous and transparent animal research. Its commitment in this direction is evident through the EQIPD framework, its quality system, and involvement in the ARRIVE 2.0 checklist. By providing free access to online resources and training, the consortium allows researchers to adopt these new quality standards, ensuring they can conduct thorough and transparent animal studies.
How to make preclinical research more effective?
For many years, applied and fundamental research in all scientific fields are facing a damning observation: the "reproducibility crisis". John Loannidis identified this phenomenon as early as 2005 (PlosMed 2005). This is a major issue, as it is estimated that over 70% of published results would not be reproducible, either by the authors themselves or by other researchers (Nature 2016). Beyond financial and human costs, this loss of rigor and efficiency slows down fundamental discoveries and innovation, to the detriment of society.
Biological and biomedical sciences are not spared from this observation, which poses a major ethical problem, as animal lives are too often wasted without any proven benefit.
The development of new drugs has significantly slowed down for more than 10 years and has decreased by 70% since 2005. The complexity of drug development is to blame, which leads to a high failure rate during the transition from preclinical phases in animals, to clinical validation in humans. To try to solve this problem, the EQIPD project, for European Quality In Preclinical Data, was supported by European funding, 4,5M€ out of 9M€, in 2017 . This initiative brought together the effort of more than 20 research groups from industry and academia for four years. In 2021, EQIPD was renamed to Enhancing Quality In Preclinical Data, and the non-profit “Guarantors of EQIPD e.V.” association was founded to ensure the sustainability and maintenance of the EQIPD resources.
EQIPD's analysis of decision-making and experimental patterns showed that there were no guiding principles or defined criteria, generally accepted and universally applicable, governing rigor in the design, conduct, and analysis of preclinical and safety research. The second observation is that scientific publications lack precision by omitting important details related to animal experimentation, which limits their usefulness to the scientific community.
The main objective of EQIPD was and remains to develop strategies to ensure that research on drug development follows better-structured processes to reinforce the robustness, rigor, and validity of data produced in preclinical studies and more broadly now for academic researchers in all studies involving animals.
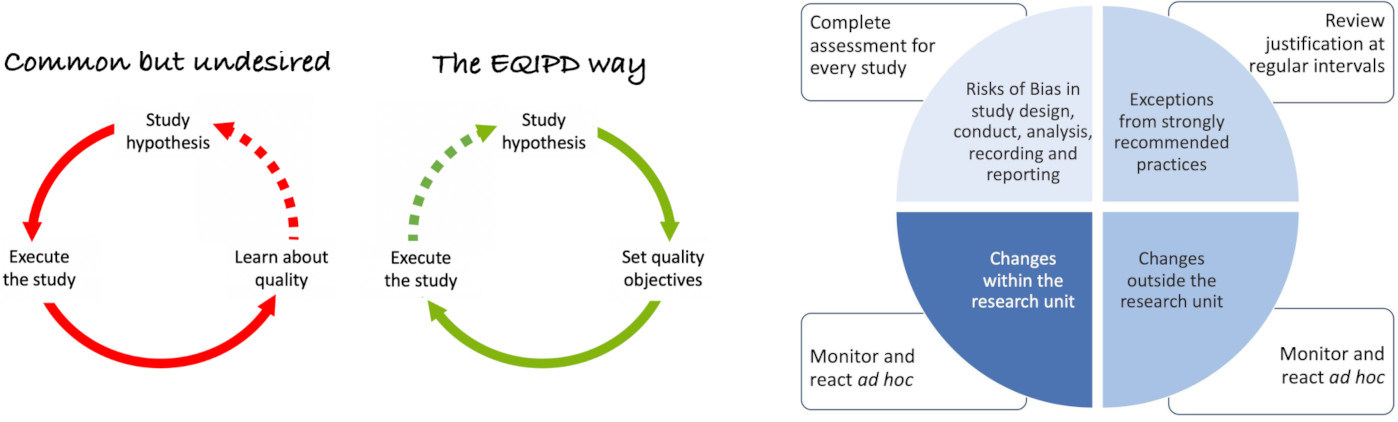
The EQIPD framework: a key standard designed for all animal studies
The EQIPD Framework is a new quality standard for designing, conducting, analyzing, and documenting animal experiments and well depicted in eLife 2021. The Guidelines for Five key areas are highlighted in NatMeth 2022:
Experiment Design: to design well-controlled experiments, use the minimum number of animals necessary, and have clear objectives and endpoints.
Statistical Analysis: to select appropriate statistical methods and reporting statistical analyses in a transparent and reproducible way.
Experiment Reporting: to report animal experiments in a transparent and complete way, including all relevant details about the experimental design, methods, and results.
Experiment Conduct: to the conduct of animal experiments, including animal welfare and ethical considerations, as well as guidelines for ensuring that the experimental procedures are conducted in a rigorous and reproducible way.
Experiment Documentation: to document all aspects of animal experiments, including the experimental design, methods, results, and any changes that were made to the experimental procedures during the course of the experiment.
An incredible numerical ecosystem
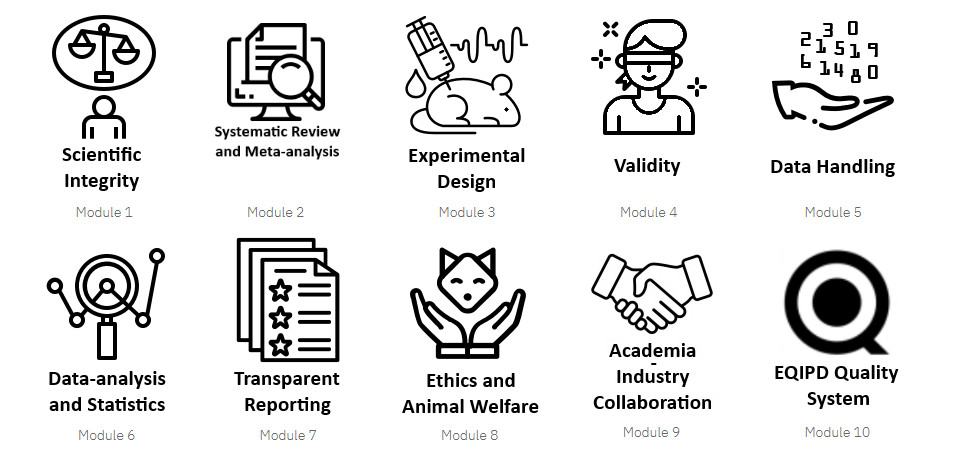
The EQIPD Quality System is a remarkable free numerical ecosystem that provides researchers with a quality management tool to implement EQIPD standards in their everyday work. This system comprises processes, procedures, and forms to ensure the quality and transparency of animal experiments. Additionally, provided on a wiki, a Toolbox help implement the EQIPD Quality System. The EQIPD initiative also offers a series of ten eLearning modules to facilitate the learning and implementation of best research practices.
EQIPD and ARRIVE 2.0 checklist
The ARRIVE 2.0 guidelines in the PlosBiol 2020 article consist in a checklist, developed by a global team of stakeholders from the scientific, publishing, and animal welfare communities. It is an updated version of the original 2010 ARRIVE guidelines. The EQIPD initiative contributed to the development of the ARRIVE 2.0 checklist by offering guidance on designing and reporting preclinical experiments, focusing on reducing bias and improving the reproducibility and translatability of research.
ARRIVE and EQIPD: proof of concept
An increasing number of scientific publications highlight the positive impact of EQIPD and ARRIVE 2.0 on animal studies :
The PlosBiol 2022 article assessed the impact of protocol harmonization on the replicability and generalizability of preclinical research findings. The study involved 7 laboratories across Europe that conducted 3 independent preclinical experiments in pain research. The results showed that harmonized protocols significantly increased the replicability and generalizability of preclinical findings across different laboratory sites. However, even after protocol harmonization, small variations in these protocols, specific to each laboratory, can introduce variability in repeated and independent studies.
Recent studies have evaluated the quality of communication of animal research results as well as the effectiveness of the updated ARRIVE 2.0 guidelines (Animals 2021, Regenerative Biomaterials 2022, PlosOne 2018). A review of 24 studies published in six high-impact journals (Animals 2021) showed that the guidelines had a positive impact on the quality of reporting. A meta-analysis of 263 publications consisting of 275 animal experiments was conducted to assess their compliance with the ten key criteria of the ARRIVE checklist (Regenerative Biomaterials 2022). The coincidence rate for the key items of the ARRIVE guidelines was only 42.0% and 41.5% for the sub-items. Improvements in certain areas, such as statistical methods and sample size calculation, are still necessary.

Overall, the EQIPD guidelines and the ARRIVE 2.0 checklist are a winning combo that has the potential to improve the quality of animal research results, reporting, experimental design, randomization, blinding, statistical analysis, as well as ethical considerations, animal welfare, and reduction of the number of animals used. However, there is still a long way to go, and further improvement, education, and training seem necessary to improve compliance with the different guidelines. Journal editors could play a major role in supporting and verifying the implementation of these recommendations (PlosOne 2018).
