The winners

Discover the 22 French researchers selected for the “Chairs of Excellence in Biology/Health" call for projects.
The "Innovation Santé 2030" plan, one of the axes of the France 2030 investment plan, launched a call for projects for "Chairs of Excellence in Biology/Health" in 2023. These chairs aim to strengthen the excellence of french biomedical research and provide funding to research teams for five years. The first 22 winners were awarded in 2024, and FC3R met one of them.
Dr. Mohamed-Ali Hakimi is a research director at Inserm and heads a team specialized in parasitology at the Institut pour l'Avancée des Biosciences (IAB) in Grenoble (Inserm, CNRS). The project led by Dr. Hakimi proposes to study the Toxoplasma gondii parasite using in vitro cell cultures, replacing the cats usually used in research to better understand infection by this parasite.

In 2000, at a time when epigenetics was in full swing, Dr. Hakimi completed a postdoctorate in gene regulation at the Wistar Institute in the USA. Fascinated by work on stem cells and their reprogramming, and with a keen interest in disease-causing viruses and parasites, Dr. Hakimi discovered toxoplasmosis at a seminar in Philadelphia. Returning to France in 2004, he established his research team with Atip-Avenir funding from Inserm and the CNRS. His team has now been working for over 20 years on the mechanisms of gene and epigenetic regulation in the context of infection by the Toxoplasma gondii parasite.
Toxoplasma gondii and toxoplasmosis
Toxoplasmosis is one of the most common parasitic zoonoses, found on every continent. The infection can affect all homeothermic (warm-blooded) vertebrates, including rodents, felines and humans.
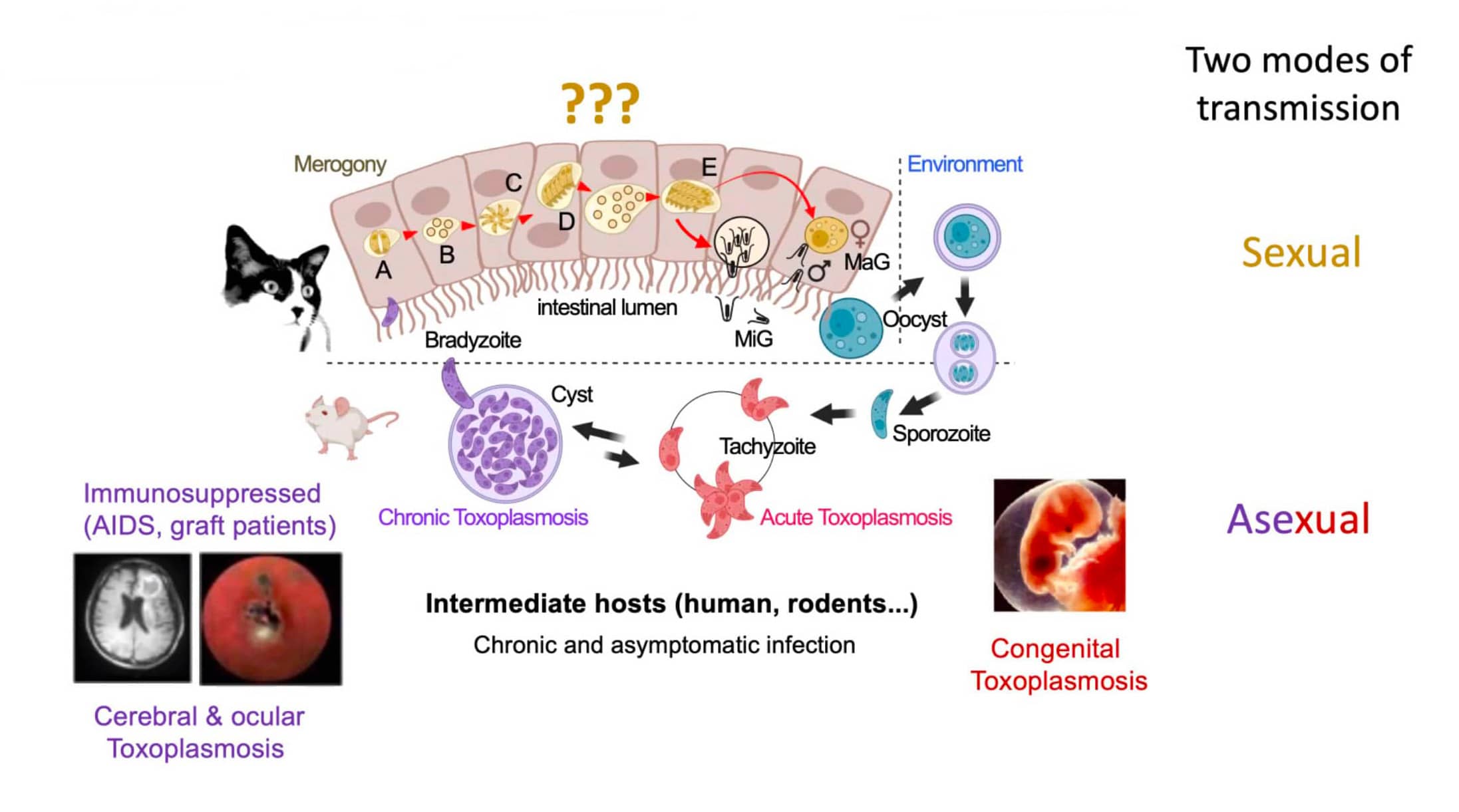
The parasite responsible for toxoplasmosis, Toxoplasma gondii, has a particularly complex life cycle, characterized by three distinct stages (all transmissible):
-
The tachyzoite, a proliferative form comparable to a stem cell, which multiplies rapidly.
-
The slow-multiplying bradyzoite forms intra-tissular or intracerebral cysts in an immunocompetent host. Rodents are the intermediate host, contaminating felins through the food chain.
-
The oocyst, resulting from sexual reproduction, develops exclusively in the intestine of felines (definitive host). They disseminate oocysts into the environment via their feces, and the soil represents a reservoir. An oocyst contains and protects around eight sporozoites (the infectious unit).
Toxoplasmosis in humans
Although humans can be infected with Toxoplasma gondii, they are an evolutionary dead end, playing no role in the transmission or maintenance of its life cycle. Humans can become infected in a number of ways: by eating raw, undercooked, or contaminated meat or poorly washed vegetables (ingestion of bradyzoites), by contact with cat feces (ingestion of sporozoites), through the placenta (mother-infant), and, more rarely, through contaminated water. In all cases, whether bradyzoite or sporozoite, the parasite develops into a tachyzoite, its active form.
In an immunocompetent patient, the pressure of the immune system encysts the parasite in its bradyzoite form, which can persist for most of the host's life. In an immunocompromised host, the bradyzoite can reactivate into the tachyzoite form, particularly in the brain, and kill the host with toxoplasmic encephalitis. Toxoplasmosis can be particularly dangerous during pregnancy (congenital toxoplasmosis) because the immaturity of the fetal immune system allows the parasite to develop in its tachyzoite form, causing abortion or severe fetal sequelae.
Studying Toxoplasma gondii: cellular models to replace cats
The sexual reproduction of Toxoplasma gondii is less well understood than its asexual reproduction (from sporozoites to bradyzoites). In the cat's intestine, the parasite develops (in the form of merozoites) in a process called merogony, producing morphotypes A, B, C, D and E, leading to the production of male gametes (microgametes) and female gametes (macrogametes).
The study of this reproduction raises ethical and technical issues related to the use of cats and other felines. To understand these mechanisms, Dr. Hakimi's team has already made a promising breakthrough without using cats. The research team used epigenetics to modify the parasite: they removed two genes that code for transcription factors. This modified parasite was then able to infect human fibroblasts in vitro, and the team was able to obtain morphotypes A, B, C, D and E, the so-called "presexual" stages (Antunes et al., 2024). However, the cycle remains blocked in vitro, preventing the production of gametes. The working hypothesis is that the parasite produces a factor (effector) that, once delivered to the host cell, would promote sexual development.
Thus, the dialog between parasite effectors and cat proteins would be necessary for the transition to the sexual stage, i.e. differentiation and sex determination. One of the team's goals is to reproduce sexual development in vitro to understand how cat proteins influence parasite proteins to change the cell environment and lead to differentiation. To understand these mechanisms, they will try to introduce into human cells certain proteins or metabolic elements that are only present in cats.
Study of domestic and wild strains
Another question the research team is addressing is the likely co-evolution of the parasite, rodents and cats with humans in the context of domestication. In fact, some strains that have evolved with humans have adapted and are not dangerous to an immunocompetent person. In a wilder environment, some strains have evolved in a "wild cycle" and can be lethal to humans. To answer this question, it is indeed important to be able to reproduce the sexual cycle in vitro to study these strains and discover virulence factors without resorting to wild cats.
Dr. Hakimi is thus enabling french parasitology to excel in its field, while at the same time replacing cats in research.
