RESEARCH TOPICS FUNDED
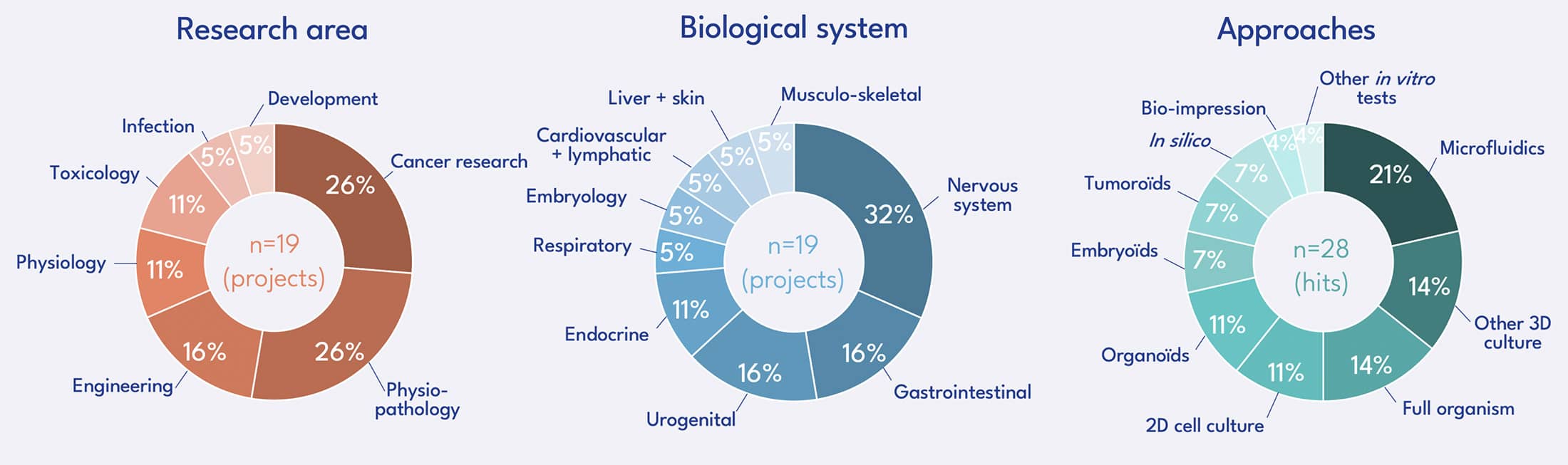
Call for projects of the FC3R – Results publication in March 2023
With this call for projects, the FC3R wished to promote innovative French research projects proposing a new strategy, method or technology favoring the Replacement - total, relative or partial - of animals and/or animal by-products used for scientific purposes: development of alternative or substitute in vitro (cell cultures, organoids, organ-on-chip), in silico (bioinformatics approaches, development of numerical models) or in chemico (biochemical toxicity studies) methods, replacement of animal by-products (fetal calf serum, extracts of basal membranes, antibodies) by synthetic and recombinant alternatives, use of cells or non-mammalian species (nematode, drosophila, zebrafish), in particular for large-scale genetic or toxicological screens, etc.
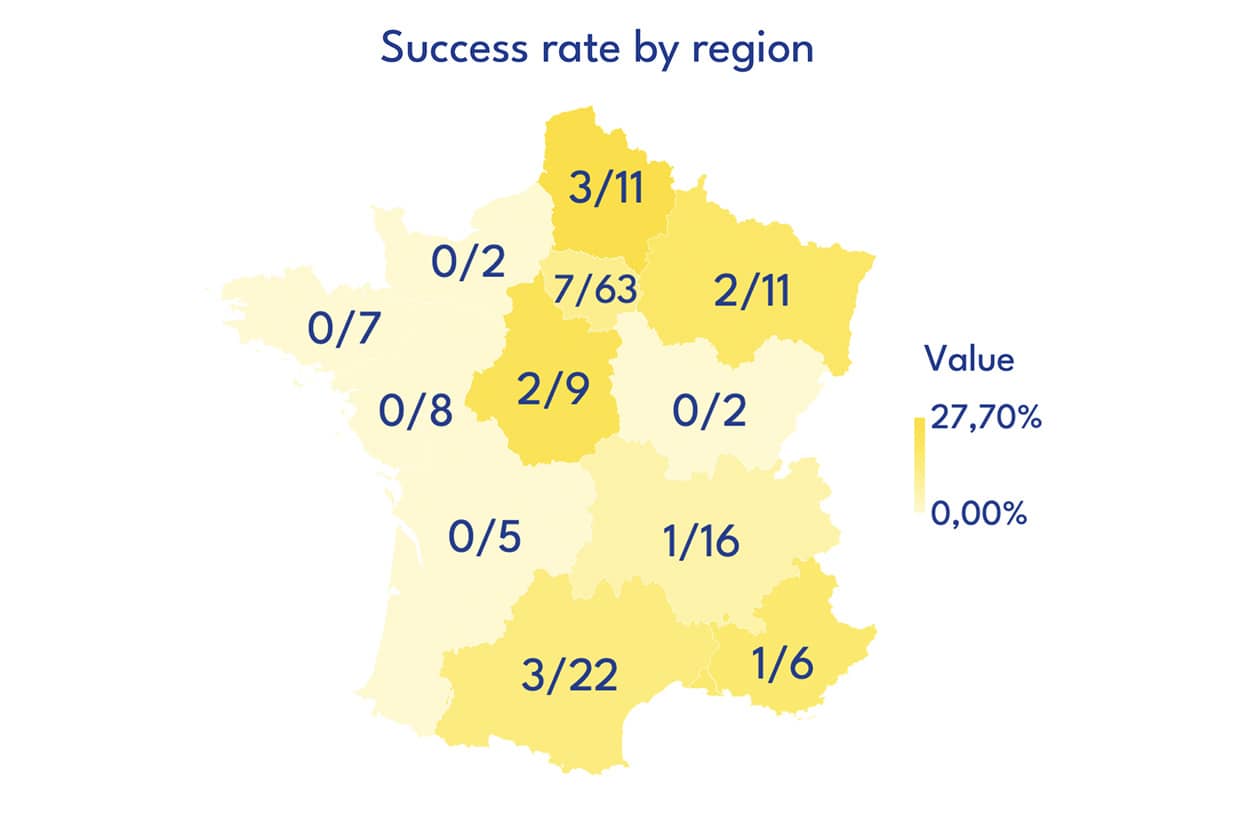
162 projects were submitted to this call. The Scientific Committee has selected 19 projects for a total funding by the FC3R of 784 467 €.

2COC : Development of complex human brain organoids to model glioblastoma studies.
Abstract:
Glioblastomas (GBM) are common brain tumors that systematically recur despite aggressive multimodal therapy. To date, the majority of studies on glioblastoma rely on intracerebral transplants of GBM cells to study its pathophysiology. Our project aims to develop an alternative to intracerebral transplants by developing cocultures of human brain organoids with glioblastoma stem cells from patients. Their complexification by vascular and immune enrichment associated with their implementation in microfluidic chips will allow to obtain a standardized cellular model mimicking the main characteristics of the tumor microenvironment of GBM. This model will be validated by comparison with data previously obtained in the laboratory. This model will eventually replace intracerebral transplants in animals and will allow the development of screening for therapeutic molecule.
The project is carried out by : Laurent GAUTHIER.
Funding allocated:
The project 2COC is allocated a funding of € 42 000.
3D-renavasc : Deciphering the molecular and functional features of the renal carcinoma-specific vasculature : Relevancy of the microtumors in 3D co-culture.
Abstract:
Clear cell renal cell carcinoma (ccRCC) is an aggressive, vascularized and metastatic tumor. The ccRCC vasculature is complex and more than 70% of the patients display partial response to treatments targeting it. We suggest that the microvessel architectural complexity interferes with the ccRCC clinical outcome. The aim of this project consists in studying the vasculature, its formation into the tumor mass and its response to treatments by using innovative in vitro tridimensional models of micro-tumors mimicking the ccRCC-specific vasculature. These models will be constituted by a complexe microenvironment and perfused, allowing long-term experiments and a pertinent drug delivery. This project will then determine the features of the ccRCC-specific vasculature, as any clinical study or animal experimentation has been able to realize, up to now.
The project is carried out by : Catherine MONNOT.
Funding allocated:
The project 3D-renavasc is allocated a funding of € 45 000.
3DMus : 3D muscle organoids.
Abstract:
The study of muscle differentiation and contraction is frequently carried out on animal models. We have recently developed a process to obtain muscle fibers from patient cells using a microfabricated device. Our project is to improve this process and make it available to researchers using animal models. By combining the efforts of biologists, biophysicists and experts in biomaterials, we act on several levels, on the one hand to use as few as possible compounds of animal origin, on the other hand to facilitate the use of this process by the largest number of researchers.
The project is carried out by : Bruno CADOT.
Funding allocated:
The project 3DMus is allocated a funding of € 45 000.
BLOOD-CROWD : Toward an organ-on-chip to mimic blood vessels in vitro: a biomimetic model of fluid extracellular macromolecular crowding.
Abstract:
Studies on vascular pathologies like atherosclerosis need the development of well established in vitro models to reduce the use of animals. In vitro models of blood vessel are already developed but none of them include the physical parameter of blood macromolecular crowding (MMC) generated by the high concentration of protein present in the blood. Here we propose to develop a new model of in vitro blood vessel which which be more reliable as it will combine vascular wall cells and blood MMC.
The project is carried out by : Rümeyza BASCETIN.
Funding allocated:
The project BLOOD-CROWD is allocated a funding of € 45 000.
BreasMo : Development of a 3D Bioprinting healthy model for reconstituting the breast tissue environment.
Abstract:
After a while, research tries to reduce the use of animals. For this, it is necessary to develop experimental models that can replace experimental animals. Our project proposes to develop a 3D model of healthy breast tissue thanks to a new and innovative technology, bioprinting. This model will be composed of a mixture of cells that make up the breast, in an attempt to mimic natural breast tissue. Once this model is developed, it could be printed in large quantities and in a very reproducible way, so it could be used in countless research topics.
The project is carried out by : Antonella RAFFO ROMERO.
Funding allocated:
The project BreasMo is allocated a funding of € 45 000.
CHICKTESTIS : A dynamic bird testis culture model.
Abstract:
Over the past years, we have observed a deterioration in the male reproductive function in humans, but also in domestic and wild species. In birds, this observation is associated with an increase in the number of endangered species. These changes constitute a societal and agronomic concern and raise questions about the links between environmental quality, breeding methods and consequences on testicular functions. At the same time, paradigm shifts in terms of animal experimentation lead us to think about the development of reduction and simplified organs called organoids which, at the testicular level, only exist to date in rodents, pigs and humans. In this project we will develop testis organoids from fetal or postnatal bird that take into account their specificity and will be relevant for the analysis of environmental variations.
The project is carried out by : Pascal FROMENT.
Funding allocated:
The project CHICKTESTIS is allocated a funding of € 36 000.
DTAD : Development of digital twin to estimate maternal pharmacokinetics and fetal exposure: example of the antidepressants.
Abstract:
For many drugs, pharmacokinetic information concerning pregnant women are missing. It is important to develop innovating tools to predict the fate of drugs without administrating them. Our project aims to create an in silico tool with data obtained from in vitro/ex vivo experiments and their physicochemical properties. To have an insight in drug transfer to the fetus, we will perfuse the human placenta. The in silico modeling enriched of data from the perfusion will allow estimating the drug quantity received by the fetus during the pregnancy. It is particularly relevant for potential toxic drugs like antidepressants that we are going to study. This methodology has the advantages to substitute the animal experimentations, to use the placenta, which is generally discarded after delivery, and to be close to the human physiology.
The project is carried out by : Frantz FOISSAC.
Funding allocated:
The project DTAD is allocated a funding of € 40 000.
Endocrinodetect : A new cell line for a myriad of specific highly sensitive in-vitro hormonal bioassays to replace in vivo assays.
Abstract:
In 2021, we published the in-vitro biological assay of a reproductive hormone (LH) from mammals (including humans) on a mouse testicular cell line. This assay measures the hormone in a single drop of blood. The reference assays are generally carried out in vivo on rodents. Our in-vitro LH assay eliminates the use of animals. By introducing the FSH receptor into these cells, we were also able to measure blood FSH as well. Our first objective is to make specific our in-vitro biological assay of FSHs . The second is to follow the same approach with other receptors to assay their corresponding hormones. The cell line rendered insensitive to LH will be made available to everyone to transfect any receptor to dose the corresponding hormone.
The project is carried out by : Yves COMBARNOUS.
Funding allocated:
The project Endocrinodetect is allocated a funding of € 26 667.
GutScreen : Development of a screening platform for Inflammatory Bowel Disease based on an environment-controlled human colon MPS.
Abstract:
Inflammatory bowel disease (IBD) is characterized by chronic inflammation, cured by drugs, and poor colon regeneration which remains a clinical challenge. Normally, intestinal stem cells (ISC) regenerate the colon. In IBD, their regenerative capacities are impaired. Today, the understanding of the tissue mechanisms involved in colon regeneration in patients remains partial. One explanation is the lack of models mimicking the characteristics of the human colon and allowing its regeneration to be monitored. New models, called 'microphysiological systems', reproduce ex-vivo with the patient's cells, the characteristics of his organ and his disease. These models reduce or even replace the use of mice in the laboratory, while improving the understanding of regeneration problems and the identification of new treatments.
The project is carried out by : Audrey FERRAND.
Funding allocated:
The project GutScreen is allocated a funding of € 45 000.
Inv-FAE-on-chip : Development of a human intestinal follicle-associated epithelium-on-chip for identifying the physiological infection portals of Shigella and Streptococcus.
Abstract:
The intestinal epithelium is one of the most important barriers delimiting the outside world from the body. In order to monitor its integrity and to fight against the invasion of pathogenic bacteria, immune system watchtowers are regularly placed along the intestine to respond more quickly to an infectious threat. However, it has been previously identified that human-specific bacteria such as Shigella and Streptococcus, usurp these defense systems in order to infect the intestinal barrier. As current study tools are not sufficiently relevant to understand the pathogenicity of these bacteria, this project will set up a new organ-on-a-chip model mimicking a complex human immunized intestine in order to identify the mechanisms underlying the infection and to provide a new technological solution allowing to reduce the use of animal models.
The project is carried out by : Alexandre GRASSART.
Funding allocated:
The project Inv-FAE-on-chip is allocated a funding of € 35 000.
IVORGA : Development of new integrated in vitro models of human organogenesis.
Abstract:
Production in labs of models of human organogenesis (organoids) represents an unprecedented opportunity to study human diseases and develop new therapeutic strategies while replacing the animal models used for these studies. Our team has recently developed a new model that mimics the early stages of neural development that controls movement and sensory information of the body, muscles, spinal bones, and certain blood vessels among others. The current project is aiming at further developing and characterizing this model while reducing the animal products needed for its production. We hope then to be able to provide research with a new integrated model of human organogenesis that will reduce the use of animals in research.
The project is carried out by : Stephane NEDELEC.
Funding allocated:
The project IVORGA is allocated a funding of € 45 000.
MILISK : Microfluidic liver-skin in vitro coculture model for toxicity studies.
Abstract:
The skin plays a major role in drug delivery and represents an important route of exposure to many xenobiotics (environmental pollutants, pesticides,..). These xenobiotics can be metabolized, absorbed and the metabolites reach the systemic circulation. As the most important organ for the metabolism of xenobiotics, the liver can also be exposed to these molecules. The objective of the MILISK project is to develop an in vitro microfluidic model for liver/skin co-culture and to mimic the physiological interactions between the two organs during skin exposure to a xenobiotic. The proposed modeml would be a powerful tool for drug development and for risk assessment of environmental pollutants. It will limit the use of animal models in accordance with the REACH regulation and the 3R rule.
The project is carried out by : Rachid JELLALI.
Funding allocated:
The project MILISK is allocated a funding of € 32 800.
MUTABRAIN : IPSC-derived human neurons and brain organoids development to study the pathophysiological impact of mutations identified in bipolar disorders.
Abstract:
Mouse models are a standard for understanding the abnormalities in brain development that underlie psychiatric disorders but modeling genetic changes and their consequences on early stages of brain development remains a real challenge. We propose here to substitute animal models by the development of neurons and brain organoid cultures derived from human cells to study the impact of mutations identified in individuals with bipolar disorder. The development of such models should help to understand the pathophysiological mechanisms underlying these disorders and these cellular models will be a powerful tool to replace animal models for testing the efficacy of new drugs.
The project is carried out by : Stéphane JAMAIN.
Funding allocated:
The project MUTABRAIN is allocated a funding of € 45 000.
NOCIPLAN : Screening of Antinociceptive and Analgesic molecules using the planarian animal model.
Abstract:
In the field of pain research most of the animals used are rodents which posit ethical and material problems. The objective of this project is therefore to develop nociceptive tests in an invertebrate, the planarian, a free living freshwater flatworm known for its regeneration abilities. We will develop new tests on acute nociception/pain but will also implement a chronic nociception/pain test where most classical treatments fail. In the long term and with automation in mind, we are hoping that this model will prevent the intense use of rodents in the pain field and will allow the development of new pain treatments.
The project is carried out by : Hervé CADIOU.
Funding allocated:
The project NOCIPLAN is allocated a funding of € 43 000.
PaSpheThIm : Characterization of heterotypic spheroid models mimicking the pancreatic tumor microenvironment for the analysis of therapeutic combinations.
Abstract:
Pancreatic cancer is particularly resistant to current therapies because of a particular configuration of the tumor which prevents immune cells from entering and playing their role of defense. One of the challenges is to identify new therapies to increase the immune cells infiltration. Such studies have until now required the use of animal models to test the effectiveness of new therapies and study their mechanisms of action. We propose in this project to develop an in vitro tumor model, called heterospheroid, composed of the main cells of the pancreatic tumor. We have demonstrated that such a model is feasible and functional and propose to validate it by studying the effect of therapeutic combinations. This project is a crucial step to drastically reduce the number of animals in preclinical experimental approaches.
The project is carried out by : Laurent GROS.
Funding allocated:
The project PaSpheThIm is allocated a funding of € 45 000.
Re-innov : Replace but innovate: a new model to understand a new side of epithelial tumorigenesis.
Abstract:
We have developed a model to study cancer in Drosophila, which has already been validated by scientific publication. We now want to show that not only does this model can replace the use of animals, but that it also allows us to answer scientific questions that are inaccessible to mice models. Our project is then to understand two poorly understood aspects of tumor life: its early stages, and the role of anti-cancer strategies in its evolution. Our preliminary results demonstrate an original response mode to a most classical treatment for breast and prostate cancer, and we now wish to understand this response with the double aim of demonstrating the scientific interest of our model, but above all of participating in the improvement of the care of cancer patients.
The project is carried out by : Cyrille DE JOUSSINEAU.
Funding allocated:
The project Re-innov is allocated a funding of € 44 000.
ROSETTE : Toward a well-defined and dynamic 3D system to study the evolution of pluripotency in vitro.
Abstract:
Pluripotent cells have the property of forming all the cells in the body. They have applications in regenerative and reproductive medicine. In the embryo, they appear and evolve in a very short developmental window. In order to avoid the use of a large number of embryos, pluripotent embryonic stem cells (ESCs) can be used. When grown in 3D in a suitable matrix, they polarise into a rosette shape, strongly mimicking what happens in the embryo during the progression of pluripotency. However, this matrix is extracted from tumours inoculated into mice. We therefore wish to test alternative matrices of non-animal origin to generate a 3D model of pluripotency with ESCs, respecting the 3Rs rule and animal welfare.
The project is carried out by : Alice JOUNEAU.
Funding allocated:
The project ROSETTE is allocated a funding of € 35 000.
SAND : Rethinking and substituting animal models of pediatric cancers: contribution of the sea aenmone Nematostella vectensis.
Abstract:
The present project focuses on the development of an innovative non-vertebrate model, the sea anemone Nematostella vectensis, to facilitate new discoveries in the field of neuronal pediatric cancers. Pediatric cancers of the central nervous system are rare malignant tumors that occur very early in life. Recent molecular data suggest that the origin of these cancers (defects during embryogenesis) is different from that of adult tumors (oncogenic mutations), largely explaining why redirected adult therapies are ineffective, as well as the absence of models pertinent. To facilitate new discoveries in the field of neural pediatric cancers, we designed a methodology involving a non-vertebrate replacement model, the sea anemone Nematostella vectensis, to test identified tumor induction candidates. Only specific candidates associated with significant scientific/medical potential , will be transferred to mammalian models (reduction).
The project is carried out by : Eric RöTTINGER.
Funding allocated:
The project SAND is allocated a funding of € 45 000.
ZFishforCFCare : Restoring host innate immunity in CF.
Abstract:
In cystic fibrosis (CF), a defective gene causes recurrent infections and an exaggerated immune response which conspire to fatally damage the lungs. In addition, the same gene defect also makes the immune system more sensitive but less effective, meaning that damage done to the lungs is caused by an overactive immune response. We have developed zebrafish as a new animal model to understand CF. Young zebrafish are transparent allowing to see the behaviour of immune cells in vivo. We will use zebrafish to understand how CF mutations are involved in unbalanced immune response in CF and test drugs that might restore immune balance. We hope this will lead to better understanding of CF and new medicines directed at the prevention of infections and immune lung damage with consequent improved survival.
The project is carried out by : Audrey BERNUT.
Funding allocated:
The project ZFishforCFCare is allocated a funding of € 45 000.
2COC : Development of complex human brain organoids to model glioblastoma studies.
3D-renavasc : Deciphering the molecular and functional features of the renal carcinoma-specific vasculature : Relevancy of the microtumors in 3D co-culture.
3DMus : 3D muscle organoids.
Abstract:
Glioblastomas (GBM) are common brain tumors that systematically recur despite aggressive multimodal therapy. To date, the majority of studies on glioblastoma rely on intracerebral transplants of GBM cells to study its pathophysiology. Our project aims to develop an alternative to intracerebral transplants by developing cocultures of human brain organoids with glioblastoma stem cells from patients. Their complexification by vascular and immune enrichment associated with their implementation in microfluidic chips will allow to obtain a standardized cellular model mimicking the main characteristics of the tumor microenvironment of GBM. This model will be validated by comparison with data previously obtained in the laboratory. This model will eventually replace intracerebral transplants in animals and will allow the development of screening for therapeutic molecule.
The project is carried out by : Laurent GAUTHIER.
Funding allocated:
The project 2COC is allocated a funding of € 42 000.
Abstract:
Clear cell renal cell carcinoma (ccRCC) is an aggressive, vascularized and metastatic tumor. The ccRCC vasculature is complex and more than 70% of the patients display partial response to treatments targeting it. We suggest that the microvessel architectural complexity interferes with the ccRCC clinical outcome. The aim of this project consists in studying the vasculature, its formation into the tumor mass and its response to treatments by using innovative in vitro tridimensional models of micro-tumors mimicking the ccRCC-specific vasculature. These models will be constituted by a complexe microenvironment and perfused, allowing long-term experiments and a pertinent drug delivery. This project will then determine the features of the ccRCC-specific vasculature, as any clinical study or animal experimentation has been able to realize, up to now.
The project is carried out by : Catherine MONNOT.
Funding allocated:
The project 3D-renavasc is allocated a funding of € 45 000.
Abstract:
The study of muscle differentiation and contraction is frequently carried out on animal models. We have recently developed a process to obtain muscle fibers from patient cells using a microfabricated device. Our project is to improve this process and make it available to researchers using animal models. By combining the efforts of biologists, biophysicists and experts in biomaterials, we act on several levels, on the one hand to use as few as possible compounds of animal origin, on the other hand to facilitate the use of this process by the largest number of researchers.
The project is carried out by : Bruno CADOT.
Funding allocated:
The project 3DMus is allocated a funding of € 45 000.
BLOOD-CROWD : Toward an organ-on-chip to mimic blood vessels in vitro: a biomimetic model of fluid extracellular macromolecular crowding.
BreasMo : Development of a 3D Bioprinting healthy model for reconstituting the breast tissue environment.
CHICKTESTIS : A dynamic bird testis culture model.
Abstract:
Studies on vascular pathologies like atherosclerosis need the development of well established in vitro models to reduce the use of animals. In vitro models of blood vessel are already developed but none of them include the physical parameter of blood macromolecular crowding (MMC) generated by the high concentration of protein present in the blood. Here we propose to develop a new model of in vitro blood vessel which which be more reliable as it will combine vascular wall cells and blood MMC.
The project is carried out by : Rümeyza BASCETIN.
Funding allocated:
The project BLOOD-CROWD is allocated a funding of € 45 000.
Abstract:
After a while, research tries to reduce the use of animals. For this, it is necessary to develop experimental models that can replace experimental animals. Our project proposes to develop a 3D model of healthy breast tissue thanks to a new and innovative technology, bioprinting. This model will be composed of a mixture of cells that make up the breast, in an attempt to mimic natural breast tissue. Once this model is developed, it could be printed in large quantities and in a very reproducible way, so it could be used in countless research topics.
The project is carried out by : Antonella RAFFO ROMERO.
Funding allocated:
The project BreasMo is allocated a funding of € 45 000.
Abstract:
Over the past years, we have observed a deterioration in the male reproductive function in humans, but also in domestic and wild species. In birds, this observation is associated with an increase in the number of endangered species. These changes constitute a societal and agronomic concern and raise questions about the links between environmental quality, breeding methods and consequences on testicular functions. At the same time, paradigm shifts in terms of animal experimentation lead us to think about the development of reduction and simplified organs called organoids which, at the testicular level, only exist to date in rodents, pigs and humans. In this project we will develop testis organoids from fetal or postnatal bird that take into account their specificity and will be relevant for the analysis of environmental variations.
The project is carried out by : Pascal FROMENT.
Funding allocated:
The project CHICKTESTIS is allocated a funding of € 36 000.
DTAD : Development of digital twin to estimate maternal pharmacokinetics and fetal exposure: example of the antidepressants.
Endocrinodetect : A new cell line for a myriad of specific highly sensitive in-vitro hormonal bioassays to replace in vivo assays.
GutScreen : Development of a screening platform for Inflammatory Bowel Disease based on an environment-controlled human colon MPS.
Abstract:
For many drugs, pharmacokinetic information concerning pregnant women are missing. It is important to develop innovating tools to predict the fate of drugs without administrating them. Our project aims to create an in silico tool with data obtained from in vitro/ex vivo experiments and their physicochemical properties. To have an insight in drug transfer to the fetus, we will perfuse the human placenta. The in silico modeling enriched of data from the perfusion will allow estimating the drug quantity received by the fetus during the pregnancy. It is particularly relevant for potential toxic drugs like antidepressants that we are going to study. This methodology has the advantages to substitute the animal experimentations, to use the placenta, which is generally discarded after delivery, and to be close to the human physiology.
The project is carried out by : Frantz FOISSAC.
Funding allocated:
The project DTAD is allocated a funding of € 40 000.
Abstract:
In 2021, we published the in-vitro biological assay of a reproductive hormone (LH) from mammals (including humans) on a mouse testicular cell line. This assay measures the hormone in a single drop of blood. The reference assays are generally carried out in vivo on rodents. Our in-vitro LH assay eliminates the use of animals. By introducing the FSH receptor into these cells, we were also able to measure blood FSH as well. Our first objective is to make specific our in-vitro biological assay of FSHs . The second is to follow the same approach with other receptors to assay their corresponding hormones. The cell line rendered insensitive to LH will be made available to everyone to transfect any receptor to dose the corresponding hormone.
The project is carried out by : Yves COMBARNOUS.
Funding allocated:
The project Endocrinodetect is allocated a funding of € 26 667.
Abstract:
Inflammatory bowel disease (IBD) is characterized by chronic inflammation, cured by drugs, and poor colon regeneration which remains a clinical challenge. Normally, intestinal stem cells (ISC) regenerate the colon. In IBD, their regenerative capacities are impaired. Today, the understanding of the tissue mechanisms involved in colon regeneration in patients remains partial. One explanation is the lack of models mimicking the characteristics of the human colon and allowing its regeneration to be monitored. New models, called 'microphysiological systems', reproduce ex-vivo with the patient's cells, the characteristics of his organ and his disease. These models reduce or even replace the use of mice in the laboratory, while improving the understanding of regeneration problems and the identification of new treatments.
The project is carried out by : Audrey FERRAND.
Funding allocated:
The project GutScreen is allocated a funding of € 45 000.
Inv-FAE-on-chip : Development of a human intestinal follicle-associated epithelium-on-chip for identifying the physiological infection portals of Shigella and Streptococcus.
IVORGA : Development of new integrated in vitro models of human organogenesis.
MILISK : Microfluidic liver-skin in vitro coculture model for toxicity studies.
Abstract:
The intestinal epithelium is one of the most important barriers delimiting the outside world from the body. In order to monitor its integrity and to fight against the invasion of pathogenic bacteria, immune system watchtowers are regularly placed along the intestine to respond more quickly to an infectious threat. However, it has been previously identified that human-specific bacteria such as Shigella and Streptococcus, usurp these defense systems in order to infect the intestinal barrier. As current study tools are not sufficiently relevant to understand the pathogenicity of these bacteria, this project will set up a new organ-on-a-chip model mimicking a complex human immunized intestine in order to identify the mechanisms underlying the infection and to provide a new technological solution allowing to reduce the use of animal models.
The project is carried out by : Alexandre GRASSART.
Funding allocated:
The project Inv-FAE-on-chip is allocated a funding of € 35 000.
Abstract:
Production in labs of models of human organogenesis (organoids) represents an unprecedented opportunity to study human diseases and develop new therapeutic strategies while replacing the animal models used for these studies. Our team has recently developed a new model that mimics the early stages of neural development that controls movement and sensory information of the body, muscles, spinal bones, and certain blood vessels among others. The current project is aiming at further developing and characterizing this model while reducing the animal products needed for its production. We hope then to be able to provide research with a new integrated model of human organogenesis that will reduce the use of animals in research.
The project is carried out by : Stephane NEDELEC.
Funding allocated:
The project IVORGA is allocated a funding of € 45 000.
Abstract:
The skin plays a major role in drug delivery and represents an important route of exposure to many xenobiotics (environmental pollutants, pesticides,..). These xenobiotics can be metabolized, absorbed and the metabolites reach the systemic circulation. As the most important organ for the metabolism of xenobiotics, the liver can also be exposed to these molecules. The objective of the MILISK project is to develop an in vitro microfluidic model for liver/skin co-culture and to mimic the physiological interactions between the two organs during skin exposure to a xenobiotic. The proposed modeml would be a powerful tool for drug development and for risk assessment of environmental pollutants. It will limit the use of animal models in accordance with the REACH regulation and the 3R rule.
The project is carried out by : Rachid JELLALI.
Funding allocated:
The project MILISK is allocated a funding of € 32 800.
MUTABRAIN : IPSC-derived human neurons and brain organoids development to study the pathophysiological impact of mutations identified in bipolar disorders.
NOCIPLAN : Screening of Antinociceptive and Analgesic molecules using the planarian animal model.
PaSpheThIm : Characterization of heterotypic spheroid models mimicking the pancreatic tumor microenvironment for the analysis of therapeutic combinations.
Abstract:
Mouse models are a standard for understanding the abnormalities in brain development that underlie psychiatric disorders but modeling genetic changes and their consequences on early stages of brain development remains a real challenge. We propose here to substitute animal models by the development of neurons and brain organoid cultures derived from human cells to study the impact of mutations identified in individuals with bipolar disorder. The development of such models should help to understand the pathophysiological mechanisms underlying these disorders and these cellular models will be a powerful tool to replace animal models for testing the efficacy of new drugs.
The project is carried out by : Stéphane JAMAIN.
Funding allocated:
The project MUTABRAIN is allocated a funding of € 45 000.
Abstract:
In the field of pain research most of the animals used are rodents which posit ethical and material problems. The objective of this project is therefore to develop nociceptive tests in an invertebrate, the planarian, a free living freshwater flatworm known for its regeneration abilities. We will develop new tests on acute nociception/pain but will also implement a chronic nociception/pain test where most classical treatments fail. In the long term and with automation in mind, we are hoping that this model will prevent the intense use of rodents in the pain field and will allow the development of new pain treatments.
The project is carried out by : Hervé CADIOU.
Funding allocated:
The project NOCIPLAN is allocated a funding of € 43 000.
Abstract:
Pancreatic cancer is particularly resistant to current therapies because of a particular configuration of the tumor which prevents immune cells from entering and playing their role of defense. One of the challenges is to identify new therapies to increase the immune cells infiltration. Such studies have until now required the use of animal models to test the effectiveness of new therapies and study their mechanisms of action. We propose in this project to develop an in vitro tumor model, called heterospheroid, composed of the main cells of the pancreatic tumor. We have demonstrated that such a model is feasible and functional and propose to validate it by studying the effect of therapeutic combinations. This project is a crucial step to drastically reduce the number of animals in preclinical experimental approaches.
The project is carried out by : Laurent GROS.
Funding allocated:
The project PaSpheThIm is allocated a funding of € 45 000.
Re-innov : Replace but innovate: a new model to understand a new side of epithelial tumorigenesis.
ROSETTE : Toward a well-defined and dynamic 3D system to study the evolution of pluripotency in vitro.
SAND : Rethinking and substituting animal models of pediatric cancers: contribution of the sea aenmone Nematostella vectensis.
Abstract:
We have developed a model to study cancer in Drosophila, which has already been validated by scientific publication. We now want to show that not only does this model can replace the use of animals, but that it also allows us to answer scientific questions that are inaccessible to mice models. Our project is then to understand two poorly understood aspects of tumor life: its early stages, and the role of anti-cancer strategies in its evolution. Our preliminary results demonstrate an original response mode to a most classical treatment for breast and prostate cancer, and we now wish to understand this response with the double aim of demonstrating the scientific interest of our model, but above all of participating in the improvement of the care of cancer patients.
The project is carried out by : Cyrille DE JOUSSINEAU.
Funding allocated:
The project Re-innov is allocated a funding of € 44 000.
Abstract:
Pluripotent cells have the property of forming all the cells in the body. They have applications in regenerative and reproductive medicine. In the embryo, they appear and evolve in a very short developmental window. In order to avoid the use of a large number of embryos, pluripotent embryonic stem cells (ESCs) can be used. When grown in 3D in a suitable matrix, they polarise into a rosette shape, strongly mimicking what happens in the embryo during the progression of pluripotency. However, this matrix is extracted from tumours inoculated into mice. We therefore wish to test alternative matrices of non-animal origin to generate a 3D model of pluripotency with ESCs, respecting the 3Rs rule and animal welfare.
The project is carried out by : Alice JOUNEAU.
Funding allocated:
The project ROSETTE is allocated a funding of € 35 000.
Abstract:
The present project focuses on the development of an innovative non-vertebrate model, the sea anemone Nematostella vectensis, to facilitate new discoveries in the field of neuronal pediatric cancers. Pediatric cancers of the central nervous system are rare malignant tumors that occur very early in life. Recent molecular data suggest that the origin of these cancers (defects during embryogenesis) is different from that of adult tumors (oncogenic mutations), largely explaining why redirected adult therapies are ineffective, as well as the absence of models pertinent. To facilitate new discoveries in the field of neural pediatric cancers, we designed a methodology involving a non-vertebrate replacement model, the sea anemone Nematostella vectensis, to test identified tumor induction candidates. Only specific candidates associated with significant scientific/medical potential , will be transferred to mammalian models (reduction).
The project is carried out by : Eric RöTTINGER.
Funding allocated:
The project SAND is allocated a funding of € 45 000.
ZFishforCFCare : Restoring host innate immunity in CF.
Abstract:
In cystic fibrosis (CF), a defective gene causes recurrent infections and an exaggerated immune response which conspire to fatally damage the lungs. In addition, the same gene defect also makes the immune system more sensitive but less effective, meaning that damage done to the lungs is caused by an overactive immune response. We have developed zebrafish as a new animal model to understand CF. Young zebrafish are transparent allowing to see the behaviour of immune cells in vivo. We will use zebrafish to understand how CF mutations are involved in unbalanced immune response in CF and test drugs that might restore immune balance. We hope this will lead to better understanding of CF and new medicines directed at the prevention of infections and immune lung damage with consequent improved survival.
The project is carried out by : Audrey BERNUT.
Funding allocated:
The project ZFishforCFCare is allocated a funding of € 45 000.